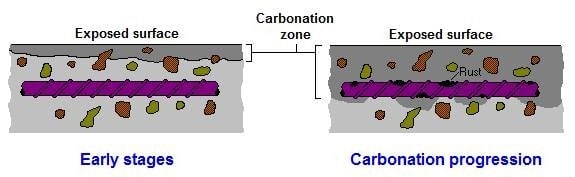
Carbonation is the single most common cause of reinforcement corrosion in above ground structures and although many construction professionals and contractors are familiar with the progression of carbonation through concrete and the consequential effects on reinforcement, some may not be familiar with research carried out in recent years. This research suggests that loss of reinforcement passivity, as the carbonation front progresses, occurs at a higher ph than previously thought.
New Concrete
The ph of new concrete is typically 12-13, which surrounds embedded reinforcement with a passivating layer of highly alkaline cement, protecting reinforcement against corrosion. The rate of carbonation in new concrete will be affected by the water/ cement ratio and the cement content; the connectivity of the capillary pore structure and size of the pores in concrete with a W/C ratio ≤ 0.4 and a cement content ≥ 400kg/m2 will be reduced when compared to concrete with a W/C ratio > 0.4 and a cement content ˂ 400kg/m2, making progress of the carbonation front slower in cement rich concrete with low W/C ratio.
The Chemical Process of Carbonation – accepted wisdom
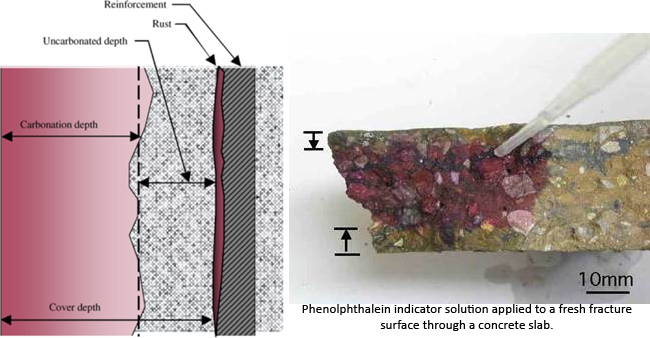
Atmospheric carbon dioxide reacts with calcium hydroxide (Ca(OH)2 + CO2 =CaCO3 + H2O), a cement hydration product in the cement paste. The reaction produces calcium carbonate. This reduces the alkalinity of the concrete to a level where the cement paste no longer provides a passive environment for embedded steel, this is said to occur when the ph of concrete falls to approximately 8.6. Steel reinforcement is then though to be susceptible to corrosion. The reaction of carbon dioxide and calcium hydroxide only occurs in solution and so in very dry concrete carbonation will be slow. In saturated concrete the moisture presents a barrier to the penetration of carbon dioxide and again carbonation will be slow. The most favourable condition for the carbonation reaction is when there is sufficient moisture for the reaction but not enough to act as a barrier. See also the later paragraph by the Concrete Society headed Loss of Passivity (Concrete Society – Carbonation of concrete)